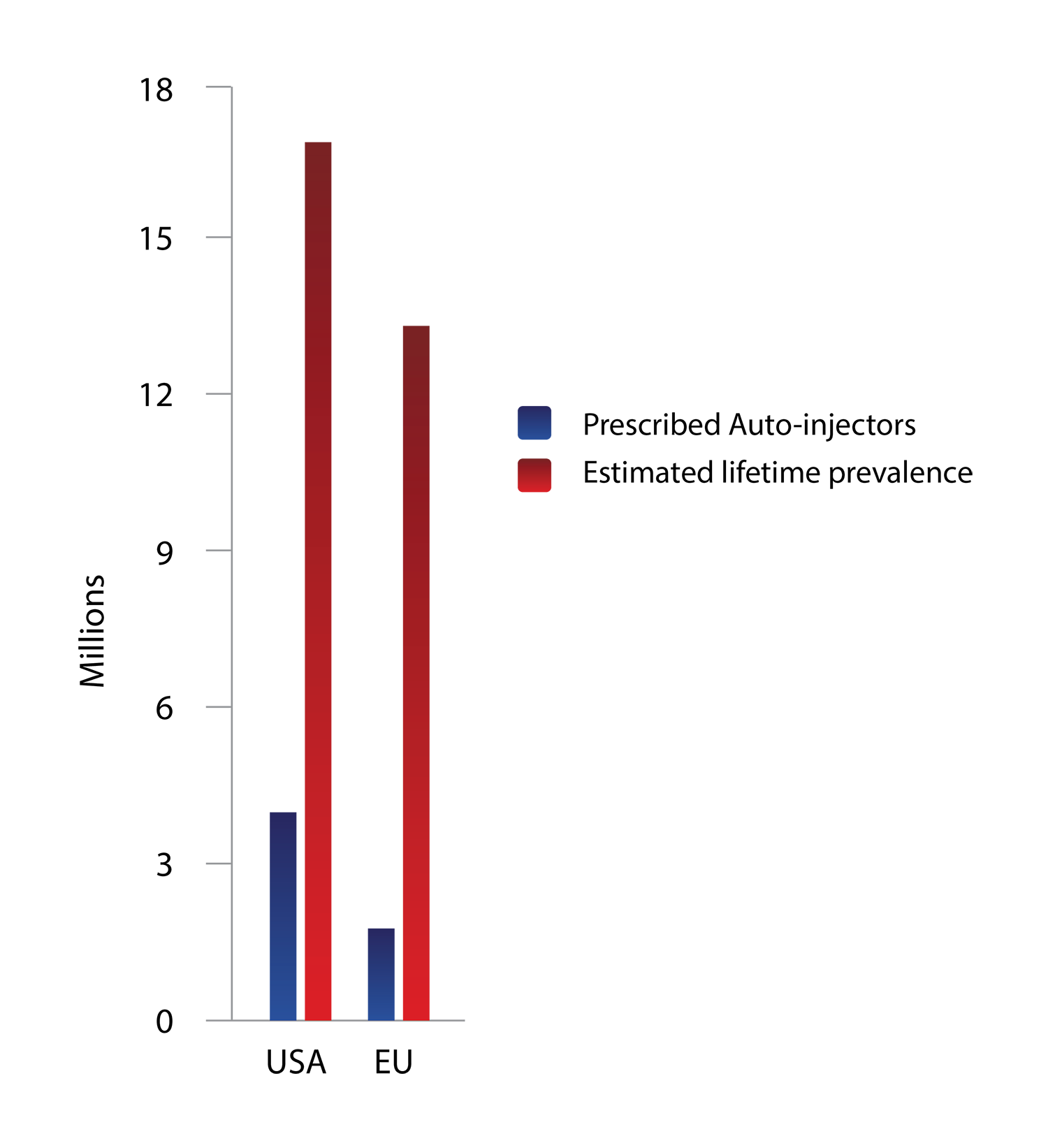
1 IN 4 PEOPLE TRY
ANOTHER APPROACH
BEFORE USING EPINEPHRINE
Among these patients, there are indicators showing that epinephrine via auto-injector is viewed as a last-resort therapy.
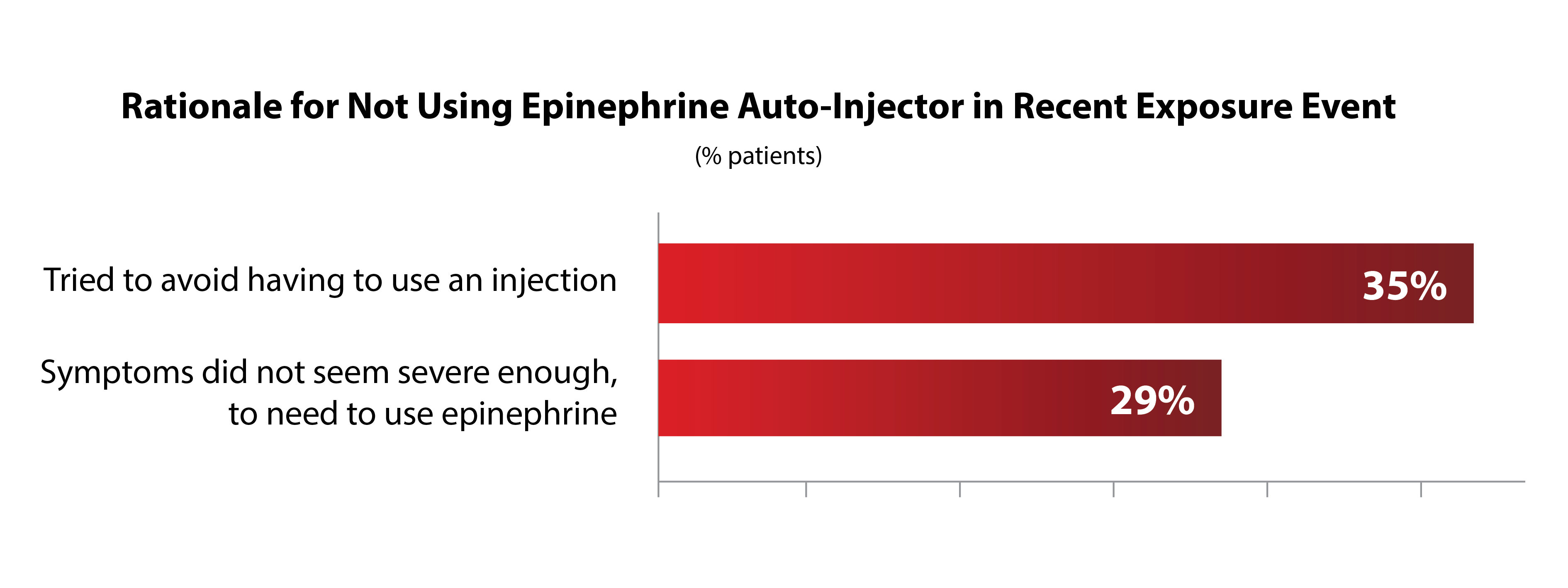
*Two most commonly given responses