BiVacor Receives Grant to Support Clinical Trials for Total Artificial Heart
TUESDAY 20 FEBRUARY, 2024
DMC offers warm congratulations to BiVACOR – a clinical-stage medical device company with world headquarters based in California and International Office based in Brisbane – on being awarded US$13 million from the Australian Government’s Medical Research Future Fund (MRFF) grant through the Artificial Heart Frontiers Program (AHFP). Driven by a desire to translate great research into better clinical outcomes, Professor John Fraser co-founded BiVACOR with its inventor Dr Daniel Timms in 2007.
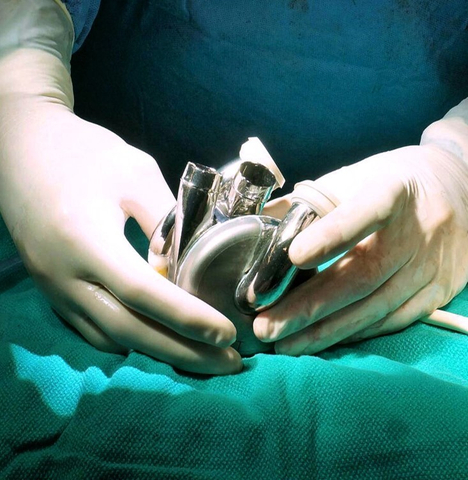
BiVacor, a clinical-stage medical device company based in Australia and California, announced an award of US$13 million from the Australian Government’s Medical Research Future Fund (MRFF) grant through the Artificial Heart Frontiers Program (AHFP). The funding will support the company’s Total Artificial Heart (TAH) program and future product enhancements.
The BiVacor TAH is an implantable total artificial heart based on rotary blood pump technology. It is designed as a long-term therapy dedicated to patients with severe biventricular heart failure. The AHFP is a consortium of research centers in Australia, led by Monash University in Melbourne, Australia, in collaboration with BiVacor.
According to the company, the US$13 million grant comes from a larger AU$50 million grant from the MRFF to the AHFP to develop and commercialize devices to treat the most common forms of severe heart failure and bring new solutions to underserved patients. The award will support clinical work of the BiVacor TAH and begin development for the integration of wireless power sources for the device.
The company advised that FDA granted an investigational device exemption for the first-in-human, early feasibility study for BiVacor’s TAH that is slated to begin in the first half of 2024. The study will include two pioneers and luminaries in cardiovascular surgery, William E. Cohn, MD, and O.H. (Bud) Frazier, MD, of the Texas Heart Institute in Houston, Texas.
“Heart failure is a chronic progressive condition in which patients suffer from debilitating symptoms, including persistent breathlessness and fatigue, that frequently require hospitalization at great cost to a patient’s quality of life and the health system,” commented Professor David Kaye, MBBS (Hons), PhD, in the company’s press release. “The average survival of a heart failure patient is comparable to some cancers at just 5 years and is even less for patients with advanced heart failure, who are the people our devices will most benefit.”
Prof. Kaye is project colead and Director of Cardiology at The Alfred in Melbourne and leads the Monash Alfred Baker Center for Cardiovascular Research.
Daniel Timms, PhD, BiVacor Founder and Chief Technology Officer, added, “There is a huge gap between available treatment options and the number of patients with severe heart failure. Initiating human clinical work for the BiVacor TAH is the first step to address critical patient needs from this noncurative disease.”
Dr. Timms continued, “We are honored to be a part of the Artificial Heart Frontiers Program, working with our close partners: lead institution Monash University, The University of New South Wales, The University of Queensland, and Griffith University, as well as our clinical partners, St. Vincent’s Hospital, Sydney, and The Alfred Hospital. The Australian government’s investment further validates the dire need for innovation in this field. It is a testament to the promise of our technology to bring these life-saving devices to market over the next few years.”
Words from Cardiac Interventions Today.
